Product Classification
- drug impurity
- drug standards
- Metaraminol bitartrate
- analytical chemistry
- standard substances
- chemical custom synthesis
- Azithromycin
- Atorvastatin
- (S)-Cinacalcet
- Ilaprazole
- Tranexamic Acid
- Duloxetine
- Octreotide
- Budesonide
- Carboprost Trometamol
- Minocycline
- Dobutamine
- Netupitant
- Axitinib
- Omadacycline
- Brivaracetam
- Metoprolol
- Indobufen
- Empagliflozin
- Dapagliflozin
- Acetylcysteine
- Hydroxychloroquine
- Tegoprazan
- Lenalidomide
- Canagliflozin
- Bromocriptine
- Rasagiline
- Bortezomib
- Diclofenac
- Bisoprolol
- Rocuronium Bromide
- Esmolol
- Bromhexine
- Probucol
- Formoterol
- Crisaborole
- Pitavastatin (3S,5S)-Isomer Calcium
- Bumetanide
- Dotinurad
- Fulvestrant
- Dotinurad
- Landiolol
Contact us
Tel:+86 17320513646
Phone:+86 17320513646
Website:https://www.moxinchem.com
Email: mike@molcoo.com
Address:Room 005, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake High-tech Development Zone, Wuhan, Hubei Province, China
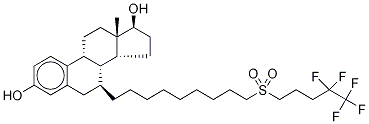
98008-06-1
- Product Name:Fulvestrant EP Impurity B
- Molecular Formula:C32H47F5O4S
- Purity:99%
- Molecular Weight:622.770196
Product Details;
CasNo: 98008-06-1
Molecular Formula: C32H47F5O4S
Manufacturer supply top purity Fulvestrant EP Impurity B 98008-06-1 with GMP standards
- Molecular Formula:C32H47F5O4S
- Molecular Weight:622.770196
- PSA:82.98000
- LogP:9.42950
Related products
-
N-Nitroso FluoxetineDetail
CAS:150494-06-7
$ -
Atorvastatin Epoxy Pyrrolooxazin 7-hydroxy analogDetail
CAS:1315629-79-8
$ -
Bumetanide EP Impurity CDetail
CAS:32643-00-8
$ -
Dotinurad ImpurityDetail
CAS:1285572-54-4
$