Product Classification
- drug impurity
- drug standards
- Metaraminol bitartrate
- analytical chemistry
- standard substances
- chemical custom synthesis
- Azithromycin
- Atorvastatin
- (S)-Cinacalcet
- Ilaprazole
- Tranexamic Acid
- Duloxetine
- Octreotide
- Budesonide
- Carboprost Trometamol
- Minocycline
- Dobutamine
- Netupitant
- Axitinib
- Omadacycline
- Brivaracetam
- Metoprolol
- Indobufen
- Empagliflozin
- Dapagliflozin
- Acetylcysteine
- Hydroxychloroquine
- Tegoprazan
- Lenalidomide
- Canagliflozin
- Bromocriptine
- Rasagiline
- Bortezomib
- Diclofenac
- Bisoprolol
- Rocuronium Bromide
- Esmolol
- Bromhexine
- Probucol
- Formoterol
- Crisaborole
- Pitavastatin (3S,5S)-Isomer Calcium
- Bumetanide
- Dotinurad
- Fulvestrant
- Dotinurad
- Landiolol
Contact us
Tel:+86 17320513646
Phone:+86 17320513646
Website:https://www.moxinchem.com
Email: mike@molcoo.com
Address:Room 005, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake High-tech Development Zone, Wuhan, Hubei Province, China
135755-51-0
- Product Name:(R,S)-Palonosetron HCl
- Molecular Formula:C19H25ClN2O
- Purity:99%
- Molecular Weight:332.8676
Product Details;
CasNo: 135755-51-0
Molecular Formula: C19H25ClN2O
Manufacturer Sells Best Quality (R,S)-Palonosetron HCl 135755-51-0 with stock
- Molecular Formula:C19H25ClN2O
- Molecular Weight:332.8676
- Melting Point:>197°C (dec.)
- PSA:23.55000
- LogP:3.33430
135755-51-0 Relevant articles
A short total synthesis of palonosetron using catalytic hydrogenation
Kowalczyk, Bruce A.
, p. 1439 - 1446 (1996)
The 5-HT3 receptor antagonists (1) and (...
Hydrochloric acid palonosetron and intermediate preparation method (by machine translation)
-
, (2018/07/07)
The invention discloses hydrochloric aci...
Preparation method of high-purity palonosetron hydrochloride
-
, (2018/08/04)
The invention discloses a preparation me...
A hydrochloric acid palonosetron production method
-
Paragraph 0054-0056, (2017/08/25)
The invention belongs to the field of or...
METHOD FOR PRODUCING HYDROBENZ ISOQUINOLINE COMPOUND
-
Paragraph 0059, (2017/04/14)
PROBLEM TO BE SOLVED: To provide a novel...
135755-51-0 Process route
-
-
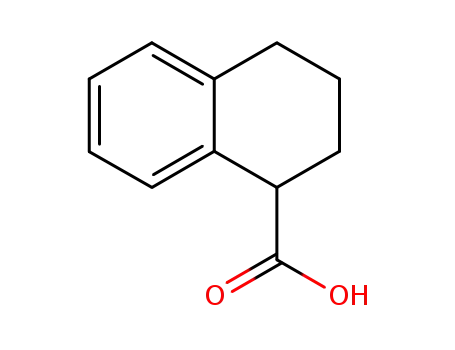
1914-65-4
tetralin-1-carboxylic acid

-
-
135755-51-0
palonosetron hydrochloride
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: N,N-dimethyl-formamide; thionyl chloride / ethyl acetate / 2.5 h / 55 °C
2.1: triethylamine / ethyl acetate / 2 h / 10 °C / Inert atmosphere
2.2: 36 h / 25 - 50 °C / Inert atmosphere
3.1: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 2.1 h / 10 °C / Reflux
4.1: toluene / 35 h / 10 - 25 °C / Inert atmosphere
4.2: 10 h / 20 °C
With
sodium tetrahydroborate; thionyl chloride; boron trifluoride diethyl etherate; triethylamine; N,N-dimethyl-formamide;
In
tetrahydrofuran; ethyl acetate; toluene;
|
|
|
Multi-step reaction with 4 steps
1.1: quinine / ethanol / 0.5 h / 0 - 50 °C
2.1: N,N-dimethyl-formamide; oxalyl dichloride / ethyl acetate / 1.05 h / 20 °C
2.2: 3 h / 50 °C
3.1: boron trifluoride diethyl etherate; sodium tetrahydroborate / tetrahydrofuran / 3 h / 15 - 65 °C
4.1: bis(trichloromethyl) carbonate / toluene / 3.5 h / Reflux
4.2: 4 h / Reflux
4.3: 2 h / Reflux
With
sodium tetrahydroborate; oxalyl dichloride; bis(trichloromethyl) carbonate; boron trifluoride diethyl etherate; quinine; N,N-dimethyl-formamide;
In
tetrahydrofuran; ethanol; ethyl acetate; toluene;
|
-
-
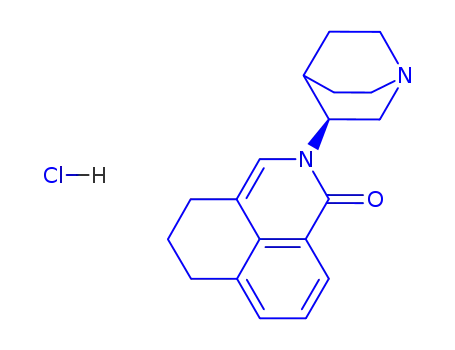
135729-55-4
(S)-2-<1-azabicyclo<2.2.2>oct-3-yl>-2,4,5,6-tetrahydro-1-oxo-1H-benz
isoquinoline hydrochloride

-

-
135755-51-0
palonosetron hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium on activated charcoal;
|
57% |
|
With
hydrogen;
palladium 10% on activated carbon;
In
methanol;
at 20 ℃;
for 24h;
under 2585.81 Torr;
|
> 99 % de |
|
With
hydrogen;
palladium on activated charcoal;
In
methanol;
at 25 - 35 ℃;
under 2585.81 Torr;
|
|
|
With
hydrogen;
palladium 10% on activated carbon;
In
propan-1-ol;
at 25 - 60 ℃;
under 2942.29 - 8091.3 Torr;
|
135755-51-0 Upstream products
-
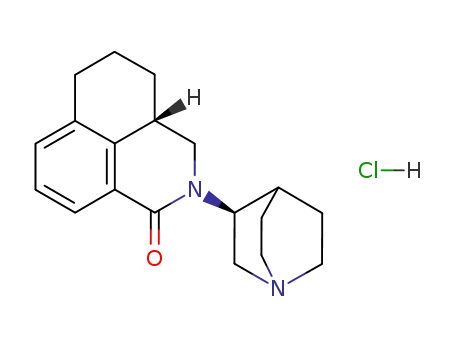
135729-55-4

(S)-2-<1-azabicyclo<2.2.2>oct-3-yl>-2,4,5,6-tetrahydro-1-oxo-1H-benz
isoquinoline hydrochloride -
1193337-96-0

2-[(S)-1-aza-bicyclo[2.2.2]oct-3-yl]-2,3,3a,4,5,6-hexahydro-1H-benz[de]isoquinolin-1-one hydrochloride
-
81-84-5
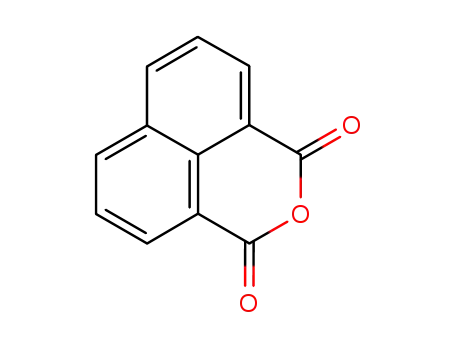
1,8-Naphthalic anhydride
-
120570-05-0
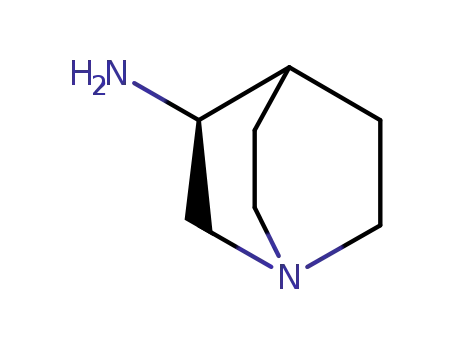
(S)-3-aminoquinuclidine
135755-51-0 Downstream products
-
135729-60-1
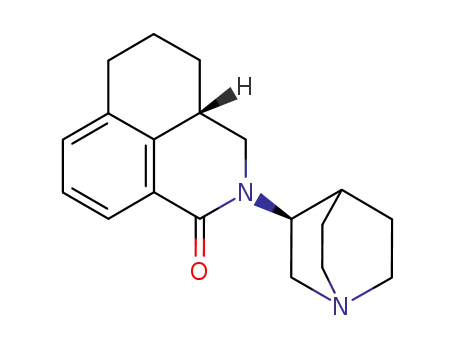
palonosetron
Related products
-
(S,R)-Palonosetron HClDetail
CAS:135729-76-9
$ -
Diclofenac EP Impurity FDetail
CAS:560075-65-2
$ -
Landiolol impurityDetail
CAS:69630-16-6
$ -
Trelagliptin impurityDetail
CAS:1108732-05-3
$