Product Classification
- drug impurity
- drug standards
- Metaraminol bitartrate
- analytical chemistry
- standard substances
- chemical custom synthesis
- Azithromycin
- Atorvastatin
- (S)-Cinacalcet
- Ilaprazole
- Tranexamic Acid
- Duloxetine
- Octreotide
- Budesonide
- Carboprost Trometamol
- Minocycline
- Dobutamine
- Netupitant
- Axitinib
- Omadacycline
- Brivaracetam
- Metoprolol
- Indobufen
- Empagliflozin
- Dapagliflozin
- Acetylcysteine
- Hydroxychloroquine
- Tegoprazan
- Lenalidomide
- Canagliflozin
- Bromocriptine
- Rasagiline
- Bortezomib
- Diclofenac
- Bisoprolol
- Rocuronium Bromide
- Esmolol
- Bromhexine
- Probucol
- Formoterol
- Crisaborole
- Pitavastatin (3S,5S)-Isomer Calcium
- Bumetanide
- Dotinurad
- Fulvestrant
- Dotinurad
- Landiolol
Contact us
Tel:+86 17320513646
Phone:+86 17320513646
Website:https://www.moxinchem.com
Email: mike@molcoo.com
Address:Room 005, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake High-tech Development Zone, Wuhan, Hubei Province, China
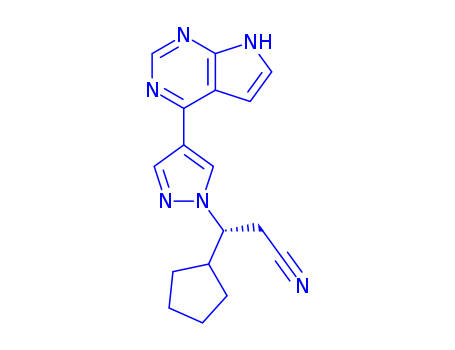
941685-37-6
- Product Name:(S)-Ruxolitinib
- Molecular Formula:C17H17N6
- Purity:99%
- Molecular Weight:306.37
Product Details;
CasNo: 941685-37-6
Molecular Formula: C17H17N6
Manufacturer supply top purity (S)-Ruxolitinib 941685-37-6 with GMP standards
- Molecular Formula:C17H18N6
- Molecular Weight:306.37
- PSA:83.18000
- LogP:3.46638
941685-37-6 Relevant articles
PROCESS AND INTERMEDIATES FOR PREPARING A JAK INHIBITOR
-
, (2022/03/04)
The present invention is related to proc...
Structural Insights into JAK2 Inhibition by Ruxolitinib, Fedratinib, and Derivatives Thereof
Davis, Ryan R.,Li, Baoli,Yun, Sang Y.,Chan, Alice,Nareddy, Pradeep,Gunawan, Steven,Ayaz, Muhammad,Lawrence, Harshani R.,Reuther, Gary W.,Lawrence, Nicholas J.,Sch?nbrunn, Ernst
supporting information, p. 2228 - 2241 (2021/03/01)
The discovery that aberrant activity of ...
PROCESS FOR PREPARING ENANTIOMERICALLY ENRICHED JAK INHIBITORS
-
, (2020/08/22)
Improved processes and intermediates for...
SYNTHESIS PROCESS OF RUXOLITINIB
-
Paragraph 0278-0280, (2019/02/05)
The present application falls within the...
941685-37-6 Process route
-
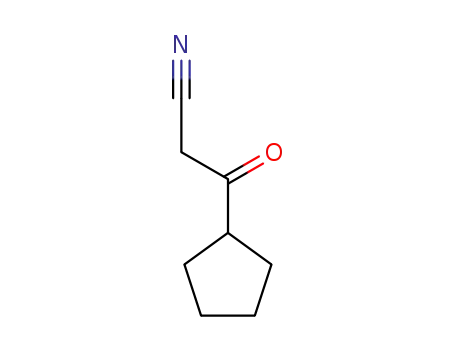
-
95882-33-0
3-cyclopentyl-3-oxopropanenitrile

-
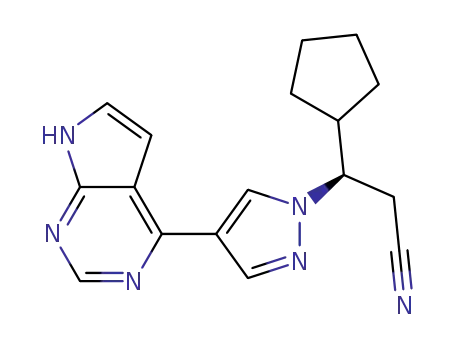
-
941678-49-5,941685-37-6
ruxolitinib
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: 4-methyl-morpholine / toluene / 0 - 5 °C / Inert atmosphere
2.1: potassium carbonate; N,N-dimethyl acetamide / water / 5 h / 23 °C
3.1: bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; hydrogen; Walphos SL-W022-1 / dichloromethane / 17 h / 7500.75 Torr / Sealed tube
4.1: trifluoroacetic acid / toluene / 0.25 h / 100 °C
4.2: 0.25 h / 40 °C
With
4-methyl-morpholine; bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; N,N-dimethyl acetamide; hydrogen; Walphos SL-W022-1; potassium carbonate; trifluoroacetic acid;
In
dichloromethane; water; toluene;
|
|
|
Multi-step reaction with 4 steps
1: 4-methyl-morpholine / toluene / 0 - 5 °C / Inert atmosphere
2: potassium carbonate; N,N-dimethyl acetamide / water / 13 h
3: bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; hydrogen; Walphos SL-W022-1 / 2,2,2-trifluoroethanol / 17 h / 20 °C / 7500.75 Torr / Sealed tube
4: trifluoroacetic acid; trifluoroacetic anhydride / toluene / 1 h / 100 °C
With
4-methyl-morpholine; bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; N,N-dimethyl acetamide; hydrogen; Walphos SL-W022-1; potassium carbonate; trifluoroacetic acid; trifluoroacetic anhydride;
In
2,2,2-trifluoroethanol; water; toluene;
|
|
|
Multi-step reaction with 4 steps
1: 4-methyl-morpholine / toluene / 0 - 5 °C / Inert atmosphere
2: potassium carbonate; N,N-dimethyl acetamide / water / 5 h
3: bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; hydrogen; Walphos SL-W022-1 / dichloromethane / 22 h / 11251.1 Torr / Sealed tube
4: phosphoric acid / isopropyl alcohol; dichloromethane / 20 °C
With
4-methyl-morpholine; bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; N,N-dimethyl acetamide; phosphoric acid; hydrogen; Walphos SL-W022-1; potassium carbonate;
In
dichloromethane; water; isopropyl alcohol; toluene;
|
-
![(3R)-3-cyclopentyl-3-[4-(7-[2-(trimethylsilyl)ethoxy]methyl-7H-pyrrolo[2,3-d]-pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile](/upload/2024/12/69d6fb46-b98a-439f-be90-bd7df5f9abac.png)
-
941685-40-1
(3R)-3-cyclopentyl-3-[4-(7-[2-(trimethylsilyl)ethoxy]methyl-7H-pyrrolo[2,3-d]-pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile

-

-
941678-49-5,941685-37-6
ruxolitinib
| Conditions | Yield |
|---|---|
|
(3R)-3-cyclopentyl-3-[4-(7-[2-(trimethylsilyl)ethoxy]methyl-7H-pyrrolo[2,3-d]-pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile;
With
boron trifluoride diethyl etherate;
In
acetonitrile;
at 60 - 70 ℃;
for 5h;
With
ammonium hydroxide; water;
In
acetonitrile;
at 20 ℃;
for 12h;
|
92.1% |
|
(3R)-3-cyclopentyl-3-[4-(7-[2-(trimethylsilyl)ethoxy]methyl-7H-pyrrolo[2,3-d]-pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile;
With
lithium tetrafluoroborate; water;
In
acetonitrile;
Inert atmosphere;
Reflux;
With
ammonia; water;
at 5 - 20 ℃;
pH=9 - 10;
optical yield given as %ee;
Inert atmosphere;
|
84% |
|
With
trifluoroacetic acid;
In
dichloromethane;
for 6h;
|
58% |
|
With
trifluoroacetic acid;
In
dichloromethane;
for 6h;
|
58% |
|
(3R)-3-cyclopentyl-3-[4-(7-[2-(trimethylsilyl)ethoxy]methyl-7H-pyrrolo[2,3-d]-pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile;
With
trifluoroacetic acid;
In
dichloromethane;
for 6h;
With
ethylenediamine;
In
methanol;
|
|
|
With
lithium tetrafluoroborate;
In
water; acetonitrile;
at 0.8 - 27 ℃;
for 11h;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 6h;
|
941685-37-6 Upstream products
-
941685-40-1

(3R)-3-cyclopentyl-3-[4-(7-[2-(trimethylsilyl)ethoxy]methyl-7H-pyrrolo[2,3-d]-pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile
-
1146629-80-2
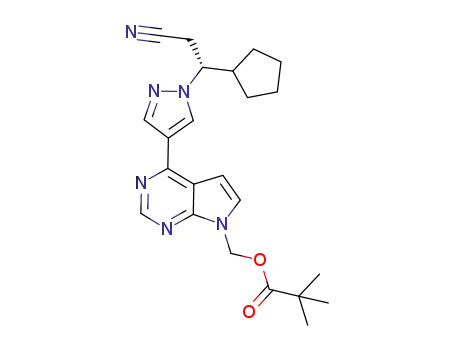
(R)-(4-(1-(2-cyano-1-cyclopentylethyl)-1H-pyrazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl pivalate
-
1146629-84-6
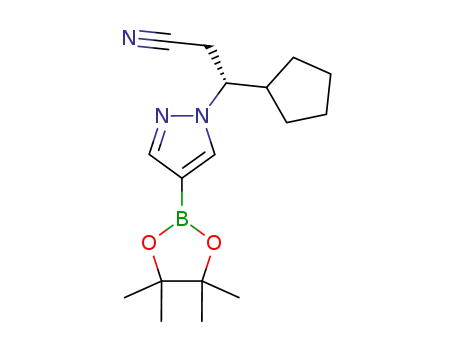
(3R)-3-cyclopentyl-3-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl]propanenitrile
-
3680-69-1
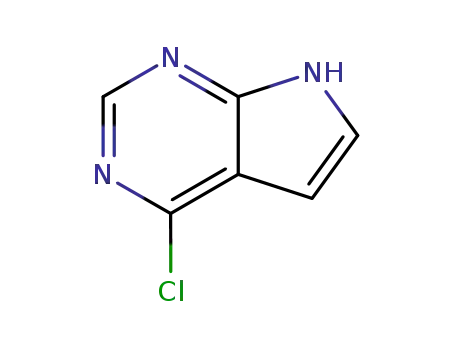
4-chloro-1H-pyrrolo[2,3-d]pyrimidine
Related products
-
(R,S)-Palonosetron HClDetail
CAS:135755-51-0
$ -
(S,R)-Palonosetron HClDetail
CAS:135729-76-9
$ -
Metaraminol bitartrate ImpurityDetail
CAS:82499-20-5
$ -
N-Nitroso FluoxetineDetail
CAS:150494-06-7
$