Product Classification
- drug impurity
- drug standards
- Metaraminol bitartrate
- analytical chemistry
- standard substances
- chemical custom synthesis
- Azithromycin
- Atorvastatin
- (S)-Cinacalcet
- Ilaprazole
- Tranexamic Acid
- Duloxetine
- Octreotide
- Budesonide
- Carboprost Trometamol
- Minocycline
- Dobutamine
- Netupitant
- Axitinib
- Omadacycline
- Brivaracetam
- Metoprolol
- Indobufen
- Empagliflozin
- Dapagliflozin
- Acetylcysteine
- Hydroxychloroquine
- Tegoprazan
- Lenalidomide
- Canagliflozin
- Bromocriptine
- Rasagiline
- Bortezomib
- Diclofenac
- Bisoprolol
- Rocuronium Bromide
- Esmolol
- Bromhexine
- Probucol
- Formoterol
- Crisaborole
- Pitavastatin (3S,5S)-Isomer Calcium
- Bumetanide
- Dotinurad
- Fulvestrant
- Dotinurad
- Landiolol
Contact us
Tel:+86 17320513646
Phone:+86 17320513646
Website:https://www.moxinchem.com
Email: mike@molcoo.com
Address:Room 005, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake High-tech Development Zone, Wuhan, Hubei Province, China
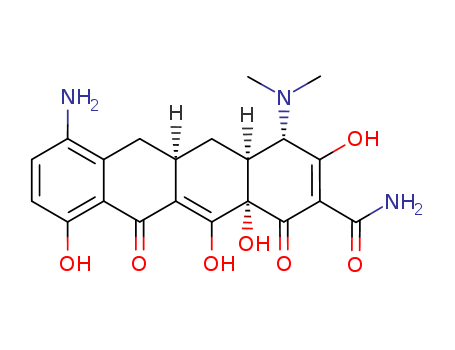
5679-00-5
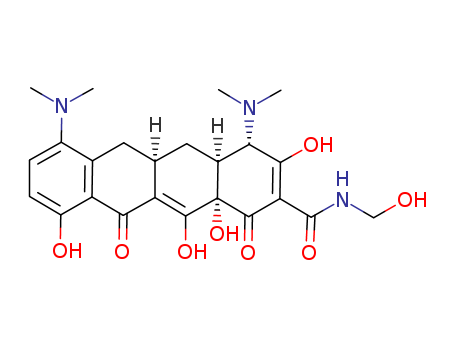
- Product Name:Minocycline EP Impurity D
- Molecular Formula:C21H23N3O7
- Purity:99%
- Molecular Weight:429.43
Product Details;
CasNo: 5679-00-5
Molecular Formula: C21H23N3O7
Factory Sells Best Quality Minocycline EP Impurity D 5679-00-5 with GMP standards
- Molecular Formula:C21H23N3O7
- Molecular Weight:429.43
- Melting Point:>213?C (dec.)
- Boiling Point:817.4±65.0 °C(Predicted)
- PKA:4.50±1.00(Predicted)
- PSA:187.41000
- Density:1.65±0.1 g/cm3(Predicted)
- LogP:0.98430
5679-00-5 Relevant articles
Preparation method of 7-amino-6-demethylation-6-deoxy tetracycline and minocycline hydrochloride
-
Paragraph 0324; 0325; 0330; 0331; 0334; 0335, (2021/04/14)
The invention relates to a preparation m...
Synthesis method of minocycline hydrochloride
-
Paragraph 0319; 0323-0325, (2021/06/21)
The invention relates to a synthesis met...
Preparation method of 7-aminominocyclin
-
Paragraph 0026-0028, (2019/10/02)
The invention discloses a preparation me...
Substituted Tetracycline Compounds
-
Page/Page column 114, (2010/12/29)
The present invention pertains, at least...
5679-00-5 Process route
-
-
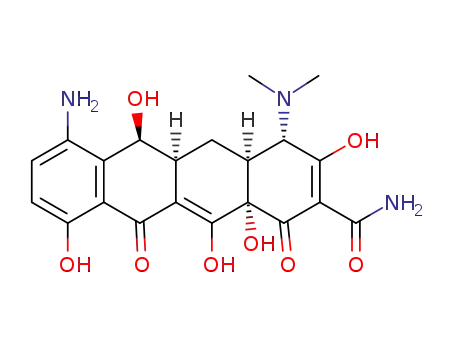
7-amino-6-demethyltetracycline

-
-
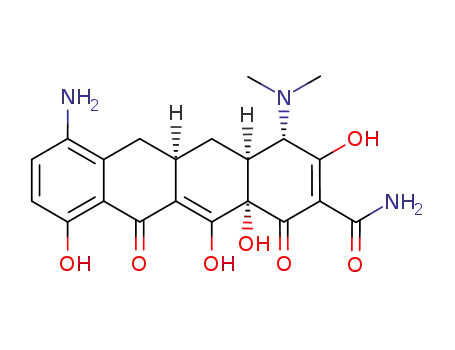
5679-00-5
7-amino-6-desmethyl-6-deoxytetracycline
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; 5% rhodium-on-charcoal; hydrogen;
In
methanol;
at 40 ℃;
for 8h;
under 18751.9 Torr;
Temperature;
Pressure;
Autoclave;
|
88.1% |
-

-
808-26-4,2696-12-0,15964-02-0,39105-46-9
sancycline

-

-
5679-00-5
7-amino-6-desmethyl-6-deoxytetracycline
| Conditions | Yield |
|---|---|
|
sancycline;
With
dibenzyl azodicarboxylate; trifluoroacetic acid;
at 5 - 10 ℃;
for 7h;
With
hydrogen;
5%-palladium/activated carbon;
In
methanol;
for 3h;
under 2068.65 Torr;
|
5679-00-5 Upstream products
-
5585-59-1
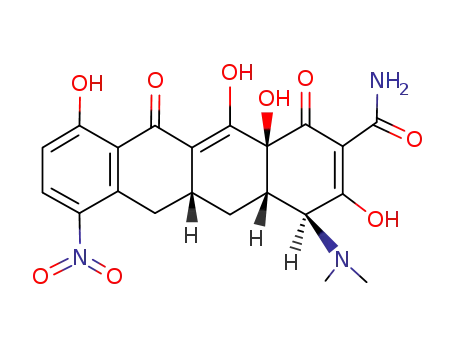
[4S-(4a,12aα)]-7-nitro-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a octahydro-naphthacene-2-carboxylic acid amide
-
808-26-4

sancycline
-
2449-05-0

dibenzyl azodicarboxylate
-
64-73-3
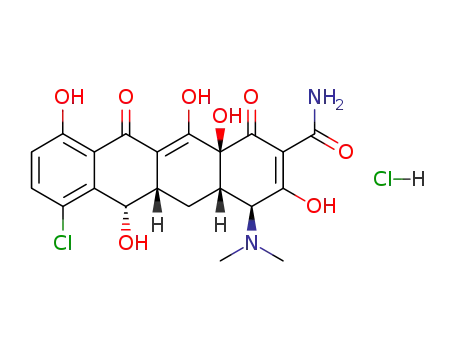
demeclocycline hydrochloride
5679-00-5 Downstream products
-
808-26-4

sancycline
-
13614-98-7
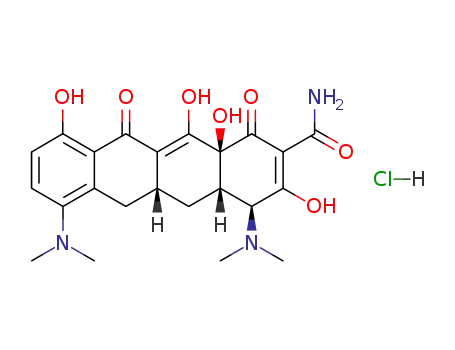
minocycline Hydrochloride
Related products
-
(R,S)-Palonosetron HClDetail
CAS:135755-51-0
$ -
(S,R)-Palonosetron HClDetail
CAS:135729-76-9
$ -
Carboprost Trometamol EP Impurity ADetail
CAS:76498-29-8
$ -
Minocycline EP Impurity FDetail
CAS:1075240-33-3
$